What is Regen® Spray Applicator?
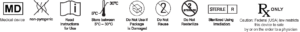
The Regen® Spray Applicator is composed of sterile and non-pyrogenic devices allowing the mix of Platelet Rich Plasma (PRP) with Autologous Serum in ratio 10:3 or 10:1.
The Spray Applicator is initially packaged in an assembled form (in the package), and must be disassembled in order to be filled. Once filled and reassembles, the Regen® Spray Applicator considerably eases the process of applying non-homogenous mixtures over greater surface areas.
Intended Use of the Device:
Regen® Spray Applicator (models R-A/NAC1 & R-A/NAC3) is designed for use in applying two non-homogenous fluids or liquids to a treatment site as deemed necessary by the clinical use requirements.
For Single Use only.
Instructions for Use (IFU):
Regen® | Innovative Preparations
Regen® Spray Applicator | R-A/NAC-1
Contains:
1 x 1 ml Luer lock syringe
2 Red transfer needles
1 x 3 ml Luer lock syringe
1 x 10 ml Luer lock syringe
1 Luer connector
1 Nozzle for spray application
1 x double piston stopper
1 x 3:10 ratio adapter
1 Applicator syringe holder
Blood Draw Accessories Compatible with 10 mL and 20 mL
RegenKit® Medical Devices are available for your convenience,
and are highly recommended to maximize preparation efficiency.





Warnings and precautions
Strict aseptic technique must be followed during the whole procedure. Use proper safety precautions to avoid contact with patient blood or cross-contamination. Use proper safety precautions to protect against needles or broken tubes. Do not use sterile component of this kit if it is opened or damaged. Do not use components of this kit if they are broken or present a defect. Do not use the tube if it has lost vacuum. Do not use the sodium citrate solution or other tube components separately. Store between 5 ºC and 30ºC; bring the kit to ambient temperature before using tubes. Do not re-sterilise, do not use after the expiration date. Single use device, do not reuse any part of the kit. Reuse may lead to infection or other illness / injury. Transfer needle must be used to transfer liquids only and should not be used for injection. The preparation of the platelet-rich plasma (PRP) must be performed by a physician trained on the equipment and procedure, or under the supervision of the physician. The treatment with PRP must be performed by a qualified physician. Do not inject PRP intravascularly. The patient must be informed of the general risks associated with the treatment and of the possible adverse effects. The safety and effectiveness of combination of PRP with other therapies should be assessed by the physician. The safety and effectiveness have not been evaluated in children and in pregnant or lactating women. The PRP must be prepared from fresh blood and must be used within four hours (extemporaneous use only).
All tubes and components of the kit are to be entirely discarded by elimination method after each use to avoid potential contamination with blood products. Use a 45° fixed angle rotor centrifuge or a horizontal head swinging bucket centrifuge (ex. RegenPRP Centri provided by Regen Lab). Follow the manufacturer’s instructions when using the centrifuge. Tubes should be centrifuged, as recommended in the instructions for use, at a relative centrifugation force (RCF) of 1500 g. Excessive RCF (over 2200 g) may lead to tube breakage resulting in blood exposure, and possible injury. RCF below 1500 g may lead to incorrect blood separation and red blood cell contamination of PRP. Centrifuge carriers and inserts size should be adapted to the tubes. Use of carriers too large or too small may result in breakage of the tubes. Caution should be taken to ensure that tubes are properly seated in the centrifuge carriers. Tubes must be balanced in the centrifuge.
Possible adverse effects
Possible side effects of blood collection
Blood collection may cause damage of the blood vessels, hematomas, superficial phlebitis, delayed wound healing, early or late infection and/or temporary or permanent nerve damage that may result in pain or numbness.
Regen® & RegenLab® are U.S. Registered trademarks of RegenLab USA and/or Regen Lab SA.
Contact Regenlab® USA
Now is the time to step into the future of medicine.
Join the Generation!
| REGENeration |
Get connected with us to Learn More.
For more information on our products, please contact us by clicking on the button below:
Our Affiliated Locations:
- New York (USA)
- Montréal (Canada)
- Venice (Italy)
- Munich (Germany)
- Paris (France)
- Dubai (U.A.E.)
- Beijing (China)
- Istanbul (Turkey)

