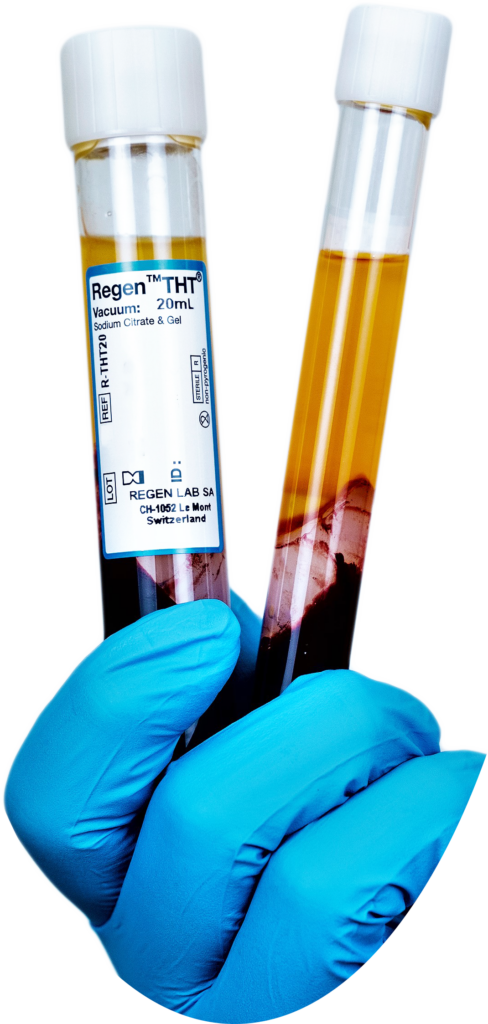
What is RegenKit® THT®?
RegenKit® THT® Medical Device Kits are available in 10 mL and 20 mL variations. They contain Regen® THT® tubes for blood collection and PRP recovery. Regen® THT® tubes are composed of pharmaceutical-grade glass with a vacuum for automated blood collection.
They contain a Sodium citrate solution and a thixotropic separating gel, to isolate plasma and platelets from other blood components and produce RegenCell® (Mononuclear-Selective A-PRP®) with a standardized composition.
The Sodium citrate anticoagulant is fully-reversible and pH neutral, lending to a considerably higher patient tolerability and enhanced ease of use.
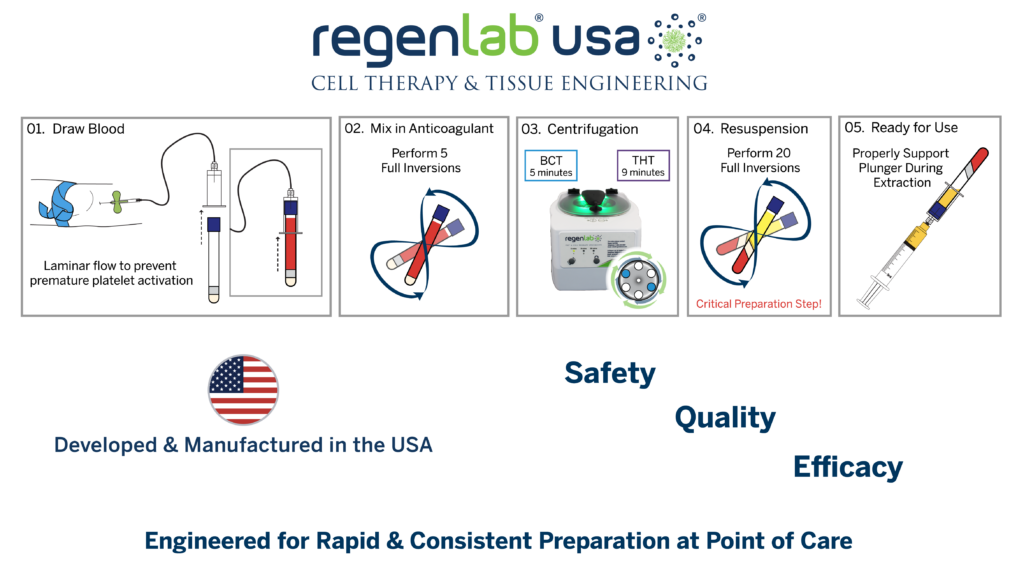
Intended Use of the Device: All models of RegenKit® THT® are designed to be used for the safe and rapid preparation of autologous platelet-rich plasma (PRP) from a small sample of blood at the patient’s point of care. The PRP is mixed with autograft and/or allograft bone prior to application to an orthopedic surgical site as deemed necessary by the clinical use requirements. RegenKit® THT® models are for single-use only.
Instructions for Use (IFU):
IFU – RegenKit THT-3 (10 mL) | IFU- RegenKit THT-3 (20 mL)
Regen® THT® Performance
The following performance tests were performed according to the US FDA requirements for platelet-rich plasma (PRP) medical devices.
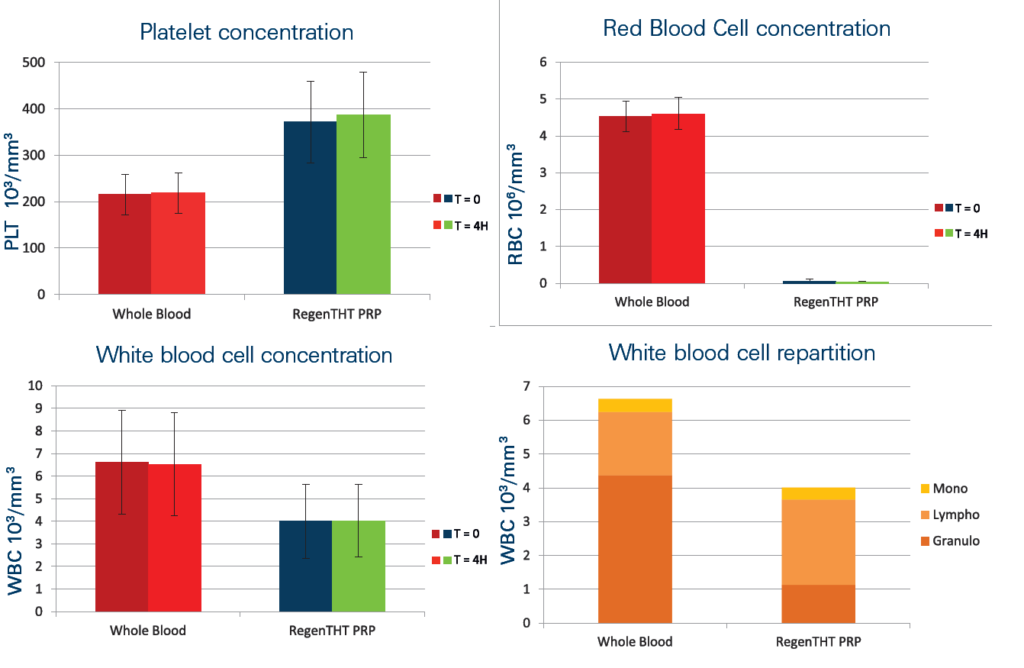
PRP was prepared with the blood from 60 volunteers (30 women and 30 men).
Measures were performed at the time of PRP preparation (T=0) and four hours later (T=4H).
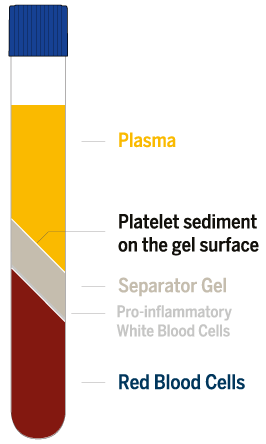
The PRP prepared with the RegenTHT device has a very low level of contaminants. Red blood cells are removed at 99.5 %.
The white blood cell level is drastically reduced, with a preferential depletion (87 %) of the pro-inflammatory granulocytes (Granulo).
The remaining white blood cells are mostly lymphocytes (Lympho) and monocytes (Mono).
The mean PRP volume obtained from 8ml of blood is 4.7 mL.
Platelet recovery is around 95 % Platelet concentration factor is 1.7 times over the whole blood value.
The flexibility of the system allows to reach a concentration factor up to 5 times (around 1’000’000 platelets/μl) by discarding a part of the plasma poor in platelets (PPP).
Regen® THT® Platelet functionality
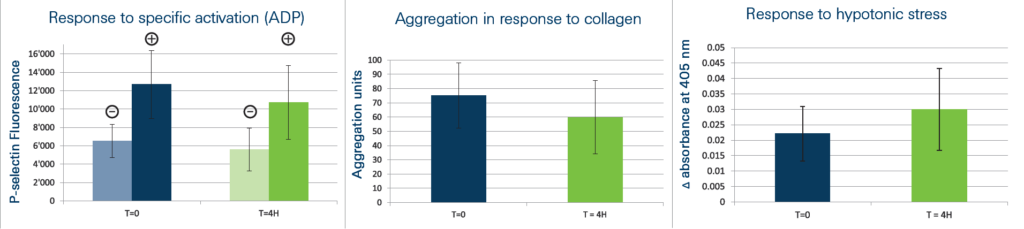
Functionality tests were done, according to the US FDA requirements, on PRP from 12 donors.
Platelets Collected with Regen® THT® are able to:
– respond to specific ADP activation by an increased expression of P-selectin
– aggregate in presence of collagen
– resist hypotonic stress
Those biological properties are maintained during the 4 hours of the experiment attesting the integrity and the functionality of the platelets.
Contrary to some competition PRP devices that don’t collect the larger platelets, there is no platelet size selection with RegenTHT tubes.
Mean platelet volume in RegenTHT PRP (7.6 ± 0.7 μm3) is similar to the value obtained in whole blood (7.6 ± 0.6 μm3)
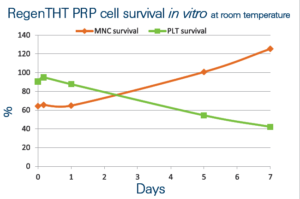
Good quality of the RegenTHT PRP is as well demonstrated by its ability to sustain MNC (mononuclear cell) growth (evaluation made in vitro in RegenTHT PRP kept at room temperature).
Platelets kept in these conditions have similar half life (7 days) as described in whole blood.
Technology platform for standardized autologous regenerative medicine
The simple, safe and efficient point-of-care preparation of autologous platelet-rich plasma
TECHNOLOGICAL ADVANTAGES
- Standardized preparation
- Minimum Blood Requirements
- Closed-Loop System: maximized safety
- Stable Physical Separation
- Reversible, Pharmaceutical-Grade Anticoagulation
- pH Neutral Anticoagulant for Patient Tolerability
- Rapid Centrifugation: 9 minutes
- Ease of Preparation: efficient & reproducible
- Facilitates and streamlines routine practice
SCIENTIFIC ADVANTAGES
- Demonstrated safety and efficacy
- Evidence-based outcomes for numerous therapeutic indications
- Large number of clinical studies, with over 200 publications
BIOLOGICAL ADVANTAGES
- Standardized, Mononuclear-Selective and Consistent
- Platelet Recovery >95%
- High Platelet Quality: viability & functionality
- Full Plasma Recovery: maximized Growth factor and fibrinogen content
- Maximized purity: >99% RBC depletion
- Mononuclear-Selective Recovery: ~70-80%
Regen® THT®
Standardized Performance. Cellular Selectivity
Available in both 10 mL and 20 mL Kits.
Regen® Innovative Preparation methods, combined with the “GMP Lab in a Tube” and the Regen® A-PRP® Cellular Selectivity Model, facilitate an efficient and consistent preparation which allows a provider to choose the preparation which best fits their individual patient’s needs.
RegenCell® | Mononuclear-Selective A-PRP®
Regen® THT® | Concentration Flexibility
Regen® | Innovative Preparations
RegenKit® THT®
RegenKit® THT®-3 (10 mL) | RK-THT-3-10
RegenKit® THT®-3 (20 mL) | RK-THT-3-20
Blood Draw Accessories Compatible with 10 mL and 20 mL
RegenKit® Medical Devices are available for your convenience,
and are highly recommended to maximize preparation efficiency.
Each RegenKit® THT®-3 contains three (3) Regen® THT® Tubes.





Warnings and precautions
Strict aseptic technique must be followed during the whole procedure. Use proper safety precautions to avoid contact with patient blood or cross-contamination. Use proper safety precautions to protect against needles or broken tubes. Do not use sterile component of this kit if it is opened or damaged. Do not use components of this kit if they are broken or present a defect. Do not use the tube if it has lost vacuum. Do not use the sodium citrate solution or other tube components separately. Store between 5 ºC and 30ºC; bring the kit to ambient temperature before using tubes. Do not re-sterilise, do not use after the expiration date. Single use device, do not reuse any part of the kit. Reuse may lead to infection or other illness / injury. Transfer needle must be used to transfer liquids only and should not be used for injection. The preparation of the platelet-rich plasma (PRP) must be performed by a physician trained on the equipment and procedure, or under the supervision of the physician. The treatment with PRP must be performed by a qualified physician. Do not inject PRP intravascularly. The patient must be informed of the general risks associated with the treatment and of the possible adverse effects. The safety and effectiveness of combination of PRP with other therapies should be assessed by the physician. The safety and effectiveness have not been evaluated in children and in pregnant or lactating women. The PRP must be prepared from fresh blood and must be used within four hours (extemporaneous use only).
All tubes and components of the kit are to be entirely discarded by elimination method after each use to avoid potential contamination with blood products. Use a 45° fixed angle rotor centrifuge or a horizontal head swinging bucket centrifuge (ex. RegenPRP Centri provided by Regen Lab). Follow the manufacturer’s instructions when using the centrifuge. Tubes should be centrifuged, as recommended in the instructions for use, at a relative centrifugation force (RCF) of 1500 g. Excessive RCF (over 2200 g) may lead to tube breakage resulting in blood exposure, and possible injury. RCF below 1500 g may lead to incorrect blood separation and red blood cell contamination of PRP. Centrifuge carriers and inserts size should be adapted to the tubes. Use of carriers too large or too small may result in breakage of the tubes. Caution should be taken to ensure that tubes are properly seated in the centrifuge carriers. Tubes must be balanced in the centrifuge.
Possible adverse effects
Possible side effects of blood collection
Blood collection may cause damage of the blood vessels, hematomas, superficial phlebitis, delayed wound healing, early or late infection and/or temporary or permanent nerve damage that may result in pain or numbness.
RegenLab®, RegenKit®, Regen®, A-PRP®, THT® & RegenCell®
are U.S. registered trademarks of RegenLab USA and/or Regen Lab SA
Contact Regenlab® USA
Now is the time to step into the future of medicine.
Join the Generation!
| REGENeration |
Get connected with us to Learn More.
For more information on our products, please contact us by clicking on the button below:
Our Affiliated Locations:
- New York (USA)
- Montréal (Canada)
- Venice (Italy)
- Munich (Germany)
- Paris (France)
- Dubai (U.A.E.)
- Beijing (China)
- Istanbul (Turkey)