“Made From You, For You”™
Regen® A-PRP® represents a unique cellular selectivity model, facilitated by Regen® innovative preparation, which not only allows for significantly different characterizations in terms of cellular presence, but also in concentration flexibility. This allows the provider to cater their preparation to the specific needs of a specific patient.
Regen® A-PRP® Cellular Selectivity
| RegenPlasma®
Leukocyte-Poor A-PRP® (RegenKit® BCT) |
RegenCell®
Monocyte-Selective A-PRP® (RegenKit® THT®) |
|||
| Platelet | Recovery | ~80% | ~95% | |
| RBC | Removal | > 99.7% | > 99% | |
| WBC | Overall | Removal | 87%-90% | 60%-70% |
| Mononuclear (Lymphocytes & Monocytes) | Recovery | 20%-30% | 70%-80%* | |
| Lymphocytes | Recovery | 25%-35% | 70%-75%* | |
| Monocytes | Recovery | ~10% | 50-55% | |
| Granulocytes | Removal | 96.5% | 85%-90% | |
Based upon performance tests carried out according to FDA requirements for platelet-rich plasma medical devices. Samples were prepared and tested using the blood of 60 volunteers (33 women and 27 men, between 18 and 67 years old).
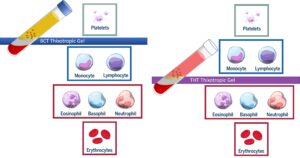
RegenKit® THT Medical Devices select for roughly 50%-60% more Mononuclear WBC’s from a whole blood sample.
Mononuclear WBC’s (Lymphocytes & Monocytes) play a role in the acute inflammatory response, but do not contribute significantly to a prolonged inflammatory response as Granulocytes do.
As such, a significant presence of Mononuclear WBC’s may be preferable, based upon the needs for a patient as dictated by the provider, so long as the overwhelming majority of Granulocytes are still removed.
Granulocyte removal between Regen® BCT and THT® only varies slightly, roughly 6.5%-11.5% based upon whole blood sample.
Other methods which claim variable selectivity require complex machinery that is both expensive and time-consuming to maintain, especially considering the disposables required for each use.
With RegenKit® Medical Devices, the technology is in the tube.
We have come to refer to this as a “GMP Lab in a Tube”
Thanks to this, our system is simple, accessible, efficient, and consistent.
Combined with Regen® innovative preparation methods and armed with leading-edge clinical research in the field of Regenerative Medicine, our provider can work with the confidence that their patients are receiving only the best in autologous cellular therapies.
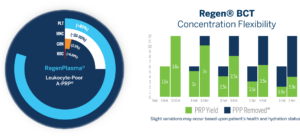
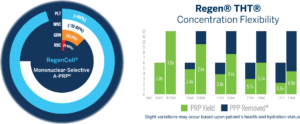
Concentration Flexibility
RegenKit® Medical Device Kits offer not only an efficient solution for selective characterization of cellular components in a preparation, but also the ability to adjust for concentration easily while maintaining exceedingly high purity of preparation and avoiding RBC infiltration.
Platelet concentration is the number of platelets per unit of volume. Usually, platelet concentration is expressed in counts (millions per mL).
However, baseline platelet concentrations and plasma yield will vary from patient to patient, based upon general health status and hydration. Due to this, It is more accurate to measure the effective recovery of our system by its concentration factor: the numerical factor by which concentration is increased.
The platelet concentration factor is the ratio between platelet concentration in PRP and baseline platelet concentration in whole blood and is generally expressed as “[n]X” or “X[n]”, where n is the value of ratio.
When all platelets are recovered in the full volume of plasma, the platelet to plasma ratio remains physiological, and the resulting platelet concentration factor is around 1.5-1.7 times the baseline value in blood (1.5-1.7X).
A physiological PRP, with recovered platelets of high quality, such as Regen® A-PRP®, is relevant for therapeutic use since it respects tissue homeostasis. The clinical results obtained with Regen® A-PRP® support this claim (more than 200 published clinical studies, lists available on request).
To get a PRP with a higher platelet concentration, either the full volume of plasma is not recovered, or a volume of the platelet-poor plasma is discarded.
Regen® BCT Recovers >80% of platelets from whole blood, with a >99.7% removal of RBC’s, and a baseline concentration of 1.6x, and a maximum concentration of 5.9x for a 10 mL draw.
Regen® THT® Recovers > 95% of platelets from whole blood, with a >99% removal of RBC’s and a baseline concentration of 1.7x to 1.8x and a maximum concentration of 6.9x for a 10 mL draw.
A growing body of research demonstrates that concentrations of platelets 1 to 3 times over baseline show more robust healing rates than those with concentrations 3 to 6 times over baseline.1
It has even been demonstrated, in some studies, that platelet concentration above a certain threshold may have negative effects. In an in-vivo study, it was shown that highly-concentrated platelet preparations had an inhibitory effect on osteoblast activity, likely due to unwanted down-regulation of desired bioactivity and cytotoxic effects of growth factors as extreme concentrations.2 Similarly, a separate in-vivo study demonstrated that platelet concentrations over 2.5x resulted in a reduction of cellular proliferation and a suboptimal effect on osteoblast functions.3
Simply put, there can be “too much of a good thing” (in terms of concentration). This is a common concept within biology. What can occur when local cellular stress occurs is a re-prioritization of biological resources and activation of compensatory mechanisms in order to re-attain homeostasis (balance) and re-establish necessary concentration gradients:
- Excretion (i.e. urinary),
- Degradation (i.e. enzyme-facilitated), or
- Transport (active and/or passive)
This can also occur in conjunction with an enhanced local immune response, which may be counterproductive and/or result in decreased patient tolerance.4,5,6
- Rappl LM et al. Effect of platelet-rich plasma gel in a physiologically relevant platelet concentration on wounds in persons with spinal cord unjury. Int Wound J 2011; 8:18.7-195.
- Weilbrich G. et al., Effect of platelet concentration in platelet-rich plasma on peri-implant bone regeneration. Bone 2004; 34:665-671
- Graziani F. et al. The in vitro effect of different PRP concentrations on osteoblasts and fibroblasts. Clin. Oral. Impl. Res. 17, 2006; 212-219.
- Fulda, S., Gorman, A. M., Hori, O., & Samali, A. (2010). Cellular stress responses: cell survival and cell death. International journal of cell biology, 2010, 214074. https://doi.org/10.1155/2010/214074
- Chen, L., Deng, H., Cui, H., Fang, J., Zuo, Z., Deng, J., Li, Y., Wang, X., & Zhao, L. (2017). Inflammatory responses and inflammation-associated diseases in organs. Oncotarget, 9(6), 7204–7218. https://doi.org/10.18632/oncotarget.23208
- Gurdon, J. B., Dyson, S., & St Johnston, D. (1998). Cells’ perception of position in a concentration gradient. Cell, 95(2), 159–162. https://doi.org/10.1016/s0092-8674(00)81747-x
RegenLab®, Regen®, RegenKit®, THT®, RegenPlasma®, RegenCell®, RegenBMC®, VetPRP®, CellularMatrix®, A-PRP® & BioBridge®
are U.S. registered trademarks of RegenLab USA and/or Regen Lab SA.
RegenWound™, RegenPRP™, RegenMatrix™, RegenARP™, ArthroVisc™, SkinVisc™, Generation Regeneration™, Made From You For You™ & RegenVET™
are U.S. pending trademarks of RegenLab USA and/or Regen Lab SA.

Contact Regenlab® USA
Now is the time to step into the future of medicine.
Join the Generation!
| REGENeration |
Get connected with us to Learn More.
For more information on our products, please contact us by clicking on the button below:
Our Affiliated Locations:
- New York (USA)
- Montréal (Canada)
- Venice (Italy)
- Munich (Germany)
- Paris (France)
- Dubai (U.A.E.)
- Beijing (China)
- Istanbul (Turkey)
