RegenKit® Wound Gel-2
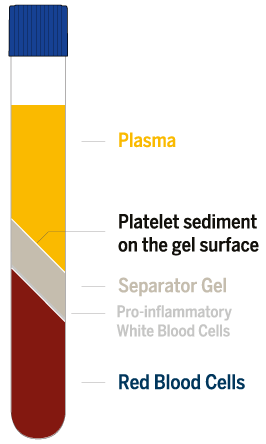
The RegenKit®-Wound Gel-2 permits autologous platelet rich plasma (PRP) and thrombin serum (ATS) to be rapidly prepared from a small volume of the patient’s blood that is drawn at the time of treatment. Blood is collected directly into RegenWound™ PRP and RegenWound™ ATS vacuum tubes and then spun in a clinical centrifuge, according to centrifuge operating instructions. Under the supervision of a healthcare professional, PRP and ATS are prepared and combined with a 10% USP calcium solution (not supplied in the kit) to produce a PRP gel (RegenWound™ | Autologous Wound Gel aka RWG), which is suitable for exuding wounds, such as leg ulcers, pressure ulcers, and diabetic ulcers, and for the management of mechanically or surgically debrided wounds.
The RegenKit®-Wound Gel-2 contains 2 RegenWound™ PRP Tubes and 1 RegenWound™ ATS Tube. Each tube is filled with 10 ml of blood (30 ml of blood needed in total) to produce approximately 13 mL of RegenWound™ Gel for use in the treatment of wounds, such as diabetic foot ulcers, classified up to 3A according to the University of Texas Classification.
Indication for Use of the Device: RegenKit®-Wound Gel-2 is designed to be used at point-of-care for the safe and rapid preparation of platelet-rich plasma (PRP) gel (RegenWound™ Gel) from a small sample of a patient’s own peripheral blood. Under the supervision of a healthcare professional, the RegenWound™ Gel is topically applied for the management of exuding cutaneous wounds, such as leg ulcers, pressure ulcers and diabetic ulcers, and mechanically or surgically debrided wounds.
RegenKit®-Wound Gel-2 should be used in conjunction with standard of care (SOC) procedures for comprehensive wound management such as: Removal of necrotic or infected tissue, Off-loading, Compression therapy for venous stasis ulcers, Establishment of adequate, blood circulation, Management of wound infection, Wound cleansing, Nutritional support, blood glucose control for patients with diabetic ulcers, Bowel/bladder care for patients with pressure ulcers at risk for contamination, Management of underlying disease or other accepted standards of care.
Instructions for Use (IFU):
Brochure:
RegenWound™ Autologous Wound Gel
RegenWound™
An Autologous Answer, to a Chronic Question
The fully biocompatible preparation of RegenWound™ capitalizes upon a synergistic coagulation process, which utilizes both autologous thrombin serum and calcium to rapidly achieve a stable gel, capable of retaining its shape and dimensions while conforming to a properly prepared wound bed.
This serves to maximize points of contact where interfacing with exposed tissue, helping to facilitate optimal diffusion and cellular communication, in a simple easy-to-prepare method, performed rapidly at point-of-care.
Usage for treatment of Non-pressure Chronic Wounds, with concomitant Diabetes Mellitus, is currently reimbursable through CMS (Centers for Medicare and Medicaid Services) under HCPCS Code G0465 for up to 20 weeks.
Reimbursement pathways for the Outpatient Hospital (POS 22 & 19) and Ambulatory Surgical (POS 24) settings follow national averages, determined by HOPPS (hospital outpatient prospective payment system). In-Office (POS 11) and Independent Clinic (POS 49) are processed through regional MACs (Medical Administrative Contractors) and vary by region.
Technology Platform for Standardized Autologous Regenerative Medicine
simple, safe and efficient point-of-care preparation of autologous platelet-rich plasma
TECHNOLOGICAL ADVANTAGES
- Standardized preparation
- Minimum Blood Requirements
- Closed-Loop System: maximized safety
- Stable Physical Separation
- Reversible, Pharmaceutical-Grade Anticoagulation
- pH Neutral Anticoagulant for Patient Tolerability
- Rapid Centrifugation: 5 minutes
- Ease of Preparation: efficient & reproducible
- Facilitates and streamlines routine practice
SCIENTIFIC ADVANTAGES
- Demonstrated safety and efficacy
- Evidence-based outcomes for numerous therapeutic indications
- Large number of clinical studies, with over 200 publications
BIOLOGICAL ADVANTAGES
- Standardized, Leukocyte-Poor, and Consistent
- Platelet Recovery >80%
- High Platelet Quality: viability & functionality
- Full Plasma Recovery: maximized Growth factor and fibrinogen content
- Maximized Purity: >99.7% RBC depletion
- Extreme Cellular Selectivity: ~96.5% GRN & 70-80% MNC elimination
RegenWound™
Comparative Effectiveness in Healing Times
Based upon study results, patients treated with RegenWound™ showed a healing completion rate over twice that of those treated with standard of care.
Clavel S, Denizot C, Boëzennec B, Turzi A, Albache NA. A randomized, controlled, clinical study comparing the efficacy of an autologous standardized leucocyte-poor platelet gel with standard care for the treatment of chronic neuropathic diabetic foot ulcers. Manuscript submitted for publication.
Regen® | Innovative Preparations
RegenKit® Wound Gel
RegenKit® Wound Gel-2 | RK-WG-2
Contains:
2 RegenWound™ PRP 10 mL Tubes
1 RegenWound™ ATS 10 mL Tube
Blood Draw Accessories Compatible with 10 mL and 20 mL
RegenKit® Medical Devices are available for your convenience,
and are highly recommended to maximize preparation efficiency.





Warnings and precautions
Strict aseptic technique must be followed during the whole procedure. Use proper safety precautions to avoid contact with patient blood or cross-contamination. Use proper safety precautions to protect against needles or broken tubes. Do not use sterile component of this kit if it is opened or damaged. Do not use components of this kit if they are broken or present a defect. Do not use the tube if it has lost vacuum. Do not use the sodium citrate solution or other tube components separately. Store between 5 ºC and 30ºC; bring the kit to ambient temperature before using tubes. Do not re-sterilise, do not use after the expiration date. Single use device, do not reuse any part of the kit. Reuse may lead to infection or other illness / injury. Transfer needle must be used to transfer liquids only and should not be used for injection. The preparation of the platelet-rich plasma (PRP) must be performed by a physician trained on the equipment and procedure, or under the supervision of the physician. The treatment with PRP must be performed by a qualified physician. Do not inject PRP intravascularly. The patient must be informed of the general risks associated with the treatment and of the possible adverse effects. The safety and effectiveness of combination of PRP with other therapies should be assessed by the physician. The safety and effectiveness have not been evaluated in children and in pregnant or lactating women. The PRP must be prepared from fresh blood and must be used within four hours (extemporaneous use only).
All tubes and components of the kit are to be entirely discarded by elimination method after each use to avoid potential contamination with blood products. Use a 45° fixed angle rotor centrifuge or a horizontal head swinging bucket centrifuge (ex. RegenPRP Centri provided by Regen Lab). Follow the manufacturer’s instructions when using the centrifuge. Tubes should be centrifuged, as recommended in the instructions for use, at a relative centrifugation force (RCF) of 1500 g. Excessive RCF (over 2200 g) may lead to tube breakage resulting in blood exposure, and possible injury. RCF below 1500 g may lead to incorrect blood separation and red blood cell contamination of PRP. Centrifuge carriers and inserts size should be adapted to the tubes. Use of carriers too large or too small may result in breakage of the tubes. Caution should be taken to ensure that tubes are properly seated in the centrifuge carriers. Tubes must be balanced in the centrifuge.
Possible adverse effects
Possible side effects of blood collection
Blood collection may cause damage of the blood vessels, hematomas, superficial phlebitis, delayed wound healing, early or late infection and/or temporary or permanent nerve damage that may result in pain or numbness.
RegenLab®, Regen® & RegenKit® are U.S. registered trademarks
of Regen Lab SA and/or RegenLab USA
RegenWound™ is a U.S. pending trademark of RegenLab USA and/or Regen Lab SA
Contact Regenlab® USA
Now is the time to step into the future of medicine.
Join the Generation!
| REGENeration |
Get connected with us to Learn More.
For more information on our products, please contact us by clicking on the button below:
Our Affiliated Locations:
- New York (USA)
- Montréal (Canada)
- Venice (Italy)
- Munich (Germany)
- Paris (France)
- Dubai (U.A.E.)
- Beijing (China)
- Istanbul (Turkey)

